Purpose: Humanized CD19 chimeric antigen receptor (CAR) T-cell therapy results in a high response rate in patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL). And many clinical trials have discovered the impact of anti-drug antibody (ADA) on CAR-T efficacy. But the ADA has yet been quantified. And its influence in patients with CAR-T infusion is still unknown.
Patients and methods: We enrolled 30 patients with R/R LBCL treated with humanized CD19 CAR-T cells between April, 2022 and July, 2023, and collected their plasma at pre-FC, day7, 14, and follow up months for ADA testing using flow cytometry.
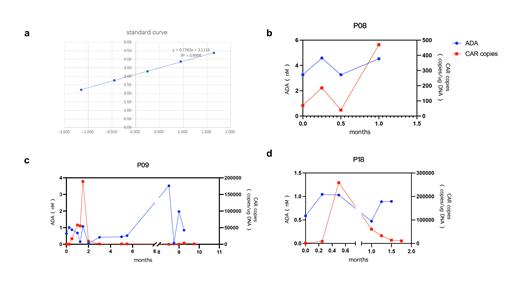
Results: The detection of ADA within plasma of humanized CAR-T was performed using flow cytometry, and the relative quantification of ADA was achieved by setting up a calibration curve with CD19 protein as a standard. R 2 of the calibration curve can be 0.999 (Figure 1a). The physiologic threshold was determined as 0.1nM (95%CI) by using plasma from 10 normal subjects.
27/30 patients who experienced only one infusion and did not relapse had mostly low ADA (<3nM). Only one exception having a maximum value higher than 5nM with PFS maintained 1 year. All of the 27 patients had ADA presence before FC, but it did not affect CAR-T amplification.
3/30 patients who received second CAR-T cell infusion, 2 of which had 2 infusions of the same product, and 1 of which relapsed after relma-cel infusion and followed by the humanized product. 2 of the 3 patients (P08 and P09) who had a high ADA before the second infusion (of 3.52 and 3.27) had extremely limited CAR-T expansion after the second infusion (maximum CAR copies of 470 and 3950, respectively), and both relapsed 1 month after the second infusion (Figure 1b-1c). The other one patient (P18) had a low ADA both before and after the second infusion (maximum is before infusion, 0.65 nM), and had normal subsequent CAR copies amplification (maximum 60800 and minimum 10850), and did not relapse during the follow-up period (Figure 1d). We consider that ADA >3nM suggests poor CAR-T amplification and poor prognosis, but further validation is needed due to the small number of patients.
Conclusions: The quantification of ADA is essential and credible, which contributes to the unification of standard. Pre-existing ADA does not affect CAR-T amplification for the first infusion but possibly suggests a negative impact on CAR-T efficacy and amplification of multiple-infusion. However, further research is needed.
Disclosures
No relevant conflicts of interest to declare.